Rhodamine B flourescent thermometry is a common technique used to measure in-channel fluid temperatures in microfluidics. However, Rhodamine B is not compatible with PDMS microchannels since the dye readily absorbs into the surrounding bulk polymer (see figure below). Absorption causes an increase in the overall fluorescent intensity which is interpreted as a false change in temperature. Many techniques that try to alleviate this problem are based on modifying the PDMS surface or incuding additives that suppress absorption to the Rhodamine B solution. However, these methods may negatively affect the intended operation of the microfluidic chip, for example, if electroosmotic flow is to be used then the zeta potential may change.
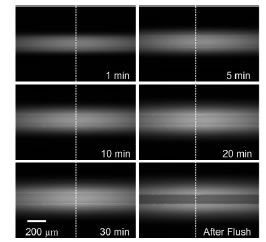
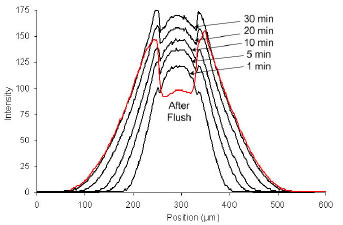
Absorption of Rhodamine B in a microchannel over a period of 30 minutes (Right) and corresponding intensity curves across the images (Left).
In this project a simple technique was developed to improve the performance of Rhodamine B thermometry measurments in PDMS chanenls. The technique is based on using photobleaching, typically an unwanted phenomenon in fluorescent thermometry, to remove the fluorescent signal emitted from absorbed Rhodamine B. Photobleaching causes a fluorphore to lose its ability to emit light after prolonged exposure to excitation light. Since absorbed Rhodamine B dye is trapped in the PDMS it can photobleached to remove the undesired signal as shown in the figure below. After removing the signal from absorbed dye, normal fluorescent thermometry images can be taken to determine the temperature field.
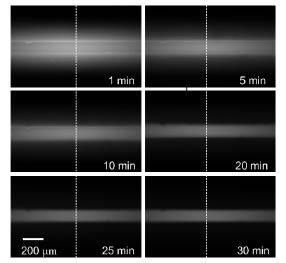
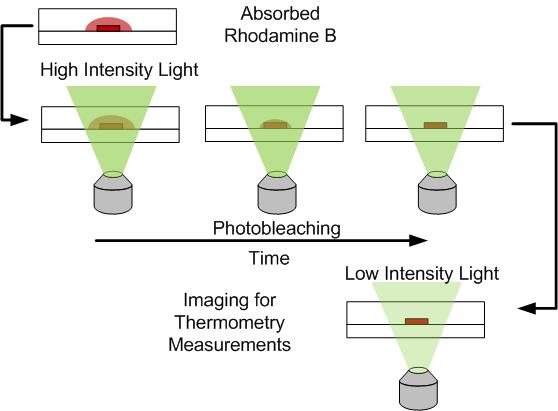
Same channel as before but time photobleaching of the area is performed using a 100W mercury arc lamp and 20X objective. A sequence of images shows the gradual decrease of absorbed Rhodamine B signal (Left). Photobleaching process diagram for obtaining thermometry images (Right).
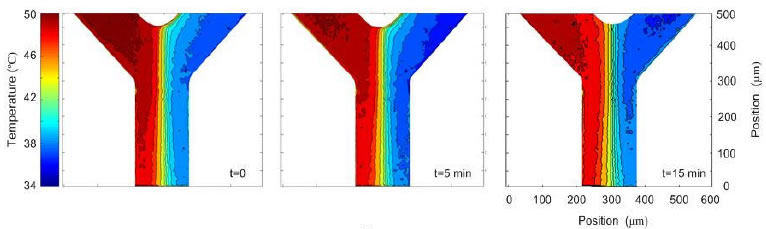
To demonstrate this technique, the steady state temperature field at the intersection of merging hot and cold streams in a Y-channel chip was studied. The figure below shows the temperature field at 0, 5 and 15 minutes. The fields are nearly identical indicating that the photobleaching technique is successful in stabilizing temperature measurements. Without photobleaching, the last result would show an erroneous 10-15°C difference compared to the first image because of the increase in fluorescent signal from absorption.
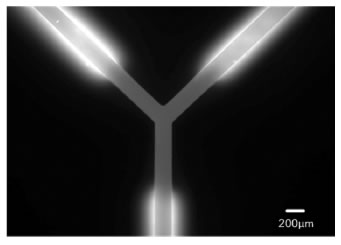
A wider view of the area shows the extent of photobleaching and the successful removal of fluorescent signal originating from absorbed Rhodamine B.
For more information on this project please refer to the following publication: